
Informações
Curriculo...
Informações para Candidatos à Orientação
Standards em Informática na Saúde
- Classificação dos Standards quanto a o que Padronizam
- Principais Standards e sua Classificação Funcional
- ISO/TC 215
- CEN/TC 251
- ABNT/CB-26
- Links para Recursos Adicionais
- Exemplos
Classificação
dos Standards quanto a o que Padronizam
Podemos classificar os Standards em:
- (MSG) Padrões de Mensagem - definem um conjunto de mensagens e sua sintaxe, como por exemplo "Cadastro de um Novo Paciente", "Dados da Reserva da Sala de Cirurgia #2 para o Paciente 12.034-X", "Dados da Reserva do Leito 2 do Apartamento 304 para o Paciente 12.034-X".
- (SRV) Padrões de Serviços - definem um Protocolo de Serviços que especifica como duas entidades (dois equipamentos, um computador e um equipamento, dois computadores, dois programas diferentes em um mesmo computador) em conformidade com o Protocolo interagem entre si e quais são os serviços que uma entidade pode prestar para a outra. Por exemplo: um software cliente de balcão de um hospital pode requisitar ao servidor de prontuário eletrônico do hospital que execute o serviço de Registrar_Um_Novo_Paciente. Em outro exemplo, uma workstation radiológica pode requistar ao aparelho de tomografia que diga Quais_Exames_Estão_Armazenados. Um bom protocolo de serviços está associado a um conjunto bem definido de mensagens, onde a cada serviço é associado um conjunto delimitado de mensagens que são trocadas entre as duas entidades durante a "negociação" do serviço.
- (STR) Documentos Estruturados - Definem uma filosofia e uma sintaxe de organização da informação em um Documento Clínico que permita: (a) sua migração entre sistemas, de forma que o prontuário de um paciente possa acompanhar o paciente, indo do PEP de um hospital para outro; (b) uma sintaxe que permita organizar a informação contida no documento em uma estrutura hierárquica que reflita o Protocolo Clínico (Algoritmo Médico) associado ao Ato Médico que gerou este documento, retirando o documento do domínio de texto puro e passando-o para o domínio das Estruturas de Dados e (c) associe aos principais termos médicos e seus modificadores códigos de alguma Terminologia Controlada de forma que os principais apsectos do conteúdo do documento sejam independentes de língua e possas sofrer tradução automática de uma língua para outra. O exemplo clássico de documento estruturado é o DICOM Structured Report criado pelo Colégio Americano de Radiologia.
- (TRM) Terminologias Controladas. Controlam o léxico utilizado para designar fenômenos e entidades da prática médica de forma a evitar mal entendidos e interpretações ambíguas e associam códigos a termos médicos de forma a tornar partes de um documento passíveis de interpretação eletrônica e independentes de lingua. Terminologias controladas costumam possuir vários Eixos de Classificação, como por exemplo Anatomia, Patologia, Processo Clínico, Farmacologia, etc. A terminologia controlada mais conhecida é o Código Internacional de Doenças (CID ver.10), que no entanto é uma terminologia extremaemnte simples pois somente apresenta um eixo de classificação, o de Patologia/Conclusão.
- (PRF) Padrões de Profilização. Tentam estandardizar os processos clínicos e organizacionais dos hospitais, definindo Workflows e Protocolos Clínicos (Algoritmos Médicos) padronizados e codificando-os, de forma a possibilitar a integração de entidades de atenção à saúde de forma a permitir que um paciente possa iniciar tratamento em um local e continuar noutro.
Standards e sua Classificação Funcional
Abaixo vai uma Lista não exaustiva dos Standards Médicos e uma associação dos mesmos a classificações de funcionalidade:
- ASTM (STR)
- CEN TC 251 / ISO TC 215 (MSG,SRV,STR,TRM). Veja também: Enterprise Workspace - ISO/TC 215 "Health informatics" onde você vai encontrar as entradas de todos os Working Groups descritos na figura abaixo.
- DeCS- Descritores em Ciências da Saúde (TRM). O vocabulário estruturado e trilingüe DeCS - Descritores em Ciências da Saúde foi criado pela BIREME para uso na indexação de artigos de revistas científicas, livros, anais de congressos, relatórios técnicos, e outros tipos de materiais, assim como para ser usado na pesquisa e recuperação de assuntos da literatura científica nas bases de dados LILACS, MEDLINE e outras.
- DICOM - Digital Image Communication in Medicine (MSG,SRV,STR)
- HL7 - Health Level Seven (MSG,SRV,STR,TRM)
- IHE - Integrating the Health Enterprise (PRF)
- ICD/CID - Código Internacional de Doenças (TRM)
- LOINC - Logical Observation Identifiers Names and Codes (TRM). The purpose of the LOINC database is to facilitate the exchange and pooling of results, such as blood hemoglobin, serum potassium, or vital signs, for clinical care, outcomes management, and research.
- MESH - Medical Subject Headings (TRM). MeSH thesaurus is a controlled vocabulary produced by the National Library of Medicine and used for indexing, cataloging, and searching for biomedical and health-related information and documents. The 2006 issue of MeSH is the printed listing of subject descriptors appearing in MEDLINE, PubMed, the NLM catalog database, and other NLM databases.
- NCPDP (MSG)
- OMG CORBAMed Medical Transcript Management (SRV) CORBA Healthcare Domain Task Force.
- RxNorm (TRM). Provides standard names for clinical drugs (active ingredient + strength + dose form) and for dose forms as administered to a patient.
- SNOMED - Sistematic Nomenclature of Medicine (TRM)
- UMLS - Unified Medical Language System (STR,TRM). The purpose of NLM's UMLS is to facilitate the development of computer systems that behave as if they "understand" the meaning of the language of biomedicine and health.
As iniciativas de normatização
ISO/TC215 e CEN/TC251 estão intimamente ligadas e são com
certeza as mais importantes para nós no Brasil, visto que as normas
ida Organização Internacional de Padronização
- ISO tem sido em geral adotadas no Brasil pela Associação
Brasileira de Normas Técnicas - ABNT após uma adaptação
às necessidades e características nacionais.
Abaixo temos uma tabela com
as normas já publicadas, os subcomitês técnicos responsáveis,
a data de publicação e um link para a página da norma,
a qual geralmente cotém um resumode seu conteúdo.
|
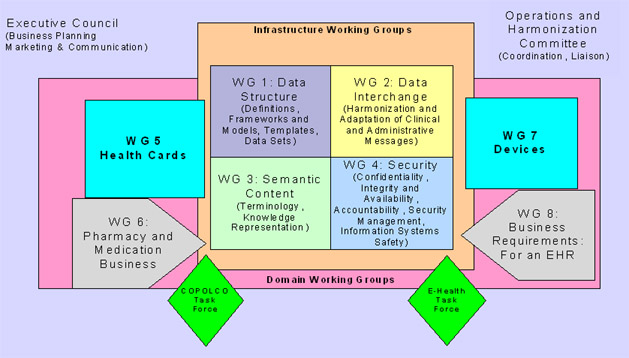
Organização
dos Working
Groups do Comitê Técnico ISO 215 numa visão de
Audrey Dickerson adickerson@himss.org
![]()
O Comité
Européen de Normalisation (CEN - European Committee for Standardization)
é interessante pois é um órgão supranacional
vinculado à União Européia que "contributes to
the objectives of the European Union and European Economic Area with voluntary
technical standards which promote free trade, the safety of workers and
consumers, interoperability of networks, environmental protection, exploitation
of research and development programmes, and public procurement. CEN was
founded in 1961 by the national standards bodies in the European Economic
Community and EFTA countries." [retirado do Scope Statement]
CEN/TC 251 Health Informatics é anterior ao Comitê ISO 215, tendo-o inspirado, e foi um comitê técnico do CEN criado para promover "standardization in the field of Health Information and Communications Technology (ICT) to achieve compatibility and interoperability between independent systems and to enable modularity. This includes requirements on health information structure to support clinical and administrative procedures, technical methods to support interoperable systems as well as requirements regarding safety, security and quality." [retirado do Scope Statement]
CEN/TC 251 é dividido, da mesma forma que o ISO/TC 215, em Grupos de Trabalho, listados abaixo:
- WG I, Modelos de Informação: "An important area of WG I work is standards for the Electronic Healthcare Record. These will include a record architecture establishing the principles for representing the information content and record structure, a set of concepts and terms for record components, and rules and mechanisms for sharing and exchanging records. A domain model representing a formal description of the context within which the healthcare records are used, will be established to document requirements for these standards. Another important area of WG I work is that of standards for messages to meet specific healthcare business needs for the communication of healthcare information. While some messages may have a broad initial scope, WG I will also validate, refine and profile these and other messages to ensure they are applicable to specialist domains with particular requirements. WG I will also address the maintenance, revision and harmonisation of existing message standards. In addition, WG I will address standards for the information requirements that may be applicable to other media for the storage and transfer of healthcare information, including patient data cards."
- WG II, Terminologia e Representação do Conhecimento: "The objectives of CEN/TC 251 WGII are the semantic organisation of information and knowledge so as to make it of practical use in the domains of health informatics and telematics and the provision of information and criteria to support harmonisation. This encompasses clinical, managerial and operational aspects of the medical record and enabling access to other knowledge."
- WG III, Segurança, Confiabilidade e Qualidade: "Major pan-European documents that provide a basis for CEN/TC 251 are the recommendations from the Council of Europe which apply to all CEN nations and the European Union Data Protection Directive finally adopted in 1995 to be implemented in the member states by October 1998. Specifications include: Secure User Identification for Healthcare — Strong Authentication using microprocessor cards; Security for Healthcare Communication; Security requirements for intermittently connected devices; Safety and Security Related Software Quality Standards for Healthcare; Framework for formal modelling of healthcare security policies; Safety procedures for identification of persons and related objects."
- WG IV, Tecnologia para Interoperabilidade: "The aim of this WG is to develop and promote standards that enable the interoperability of devices and information systems in health informatics. The scope covers three main areas: (1) Intercommunication of data between devices and information systems; (2) Integration of data for multimedia representation; (3) Communication of such data between source departments and other legitimate users elsewhere in the healthcare sector, in order to facilitate electronic healthcare record provision"
- Task Force Cards: Health Cards. Revision of ENV 12018 together with ISO/TC 215 (Frans Van Bommel)
- Task Force HISA, Revision of ENV 12967: Health informatics — Service architecture. Part 1: Enterprise viewpoint; Part 2: Information viewpoint; Part 3: Computational viewpoint. (Gunnar Klein)
- Task Force EHRcom, Revision of ENV 13606: .Electronic Health Record Communication. Part 1: Extended architecture; Part 2: Domain termlist; Part 3: Distribution rules; Part 4: Message for the exchange of information
- PT-26 Electronic Healthcare Record Communication - Part 1: Extended Architecture and Domain Model (Stephen Kay)
- PT-27 Electronic Healthcare Record Communication - Part 2: Domain Termlist (Angelo Rossi Mori)
- PT-28 Electronic Healthcare Record Communication - Part 3: Distribution Rules (Robin Hopkins)
- PT-29 Electronic Healthcare Record Communication -Part 4: Messages for the exchange of information (David Markwell)
- PT-30 System of Concepts to Support Continuity of Care (Francois Mennerat)
- PT-31 Messages for the Exchange of Information on Drug Prescription (Jesper Theilgaard)
- PT-32 Blood Transfusion Related Messages (Christian Desaint)
- PT-33 Messages for Maintenance of Supporting Information in Healthcare Systems (Niels Jørgen Christensen
- PT-34 Interoperability of Healthcare Multimedia Report Systems (Nicholas Brown)
- PT-35 Interoperability of Medical Devices within Acute Care Units (Paul Woolman)
- PT-36 Instrument Interfaces to Laboratory Information Systems (Richard Hayes)
- PT-37 Secure User Identification for Healthcare — Strong Authentication using Microprocessor Cards (Lionel Moss)
- PT-38 Safety and Security related Software Quality Standards (Paul Wardman)
- PT-39 Security for Healthcare Communication (Tor Olav Grøtan)
- PT-40 File exchange format for Vital Signs (Alpo Värri)
- PT-41 General Purpose Information Components (Gunnar Klein)
- PT-42 Service Request and Report Messages (Edgar Gl|ck)
PT-44 Mapping of Hierarchical Message Descriptions to XML (Yves Mounier)
|
ABNT - Associação Brasileira de Normas Técnicas
A ABNT é o órgão oficial de normatização no Brasil. Todo o trabalho realizado pela ABNT nos Comitês Brasileiros e Organismos de Normalização Setorial é orientado para atender ao desenvolvimento da tecnologia e participação efetiva na normalização internacional e regional. A Comissão de Estudo Especial Temporária (CEET) da ABNT é uma Comissão de Estudo vinculada à Gerência do Processo de Normalizacão da ABNT, com objetivo e prazo determinados, para tratar do assunto não coberto pelo âmbito de atuação dos Comitês Técnicos. O Comitê Brasileiro (ABNT/CB) é um órgão da estrutura da ABNT com Superintendente eleito pelos sócios da ABNT, nele inscritos, com mandato de 2 anos, permitidas duas reeleições.
A ABNT possui atualmente
53 Comitês e 3 Organismos de Normalização Setorial.
Na área da Saúde temos o Comitê ABNT/CB-26 - Odonto
Médico Hospitalar atuando nas seguintes áreas: Normalização
no campo odonto-médico-hospitalar compreendendo produtos correlatos
de saúde tais como: materiais, artigos, aparelhos, dispositivos,
instrumentos e acessórios cujo uso ou aplicação na
prática médica, hospitalar, odontológica e de laboratório
estejam associados às ações e serviços de saúde,
no que concerne a
terminologia, requisitos,
métodos de ensaio e generalidades. Excluindo-se a normalização
de radiação não-ionizante que é de responsabilidade
do ABNT/CB-20. Na área da Informática Médica
ainda (Março de 2006) não existem Comitês atuantes
no Brasil, isso podendo ser observado ao ser analisar de quais comitês
ISO o CB-26 participa.
Superintendenter: Luiz Fernando
de Andrade Viotti
Secretaria Técnica:
ABIMO - Associação Brasileira da Indústria de
Artigos e Equipamentos Médicos,
Odontológicos, Hospitalares e
de Laboratórios
Chefe de Secretaria: Rita
de Cássia Cação
Av. Paulista, 1313 - 8º
andar - Sala 806
Cep: 01311-923 - São
Paulo - SP
Fone: (11) 3285-0155 ramal
32
Fax: (11) 3285-0155 ramal
30
E-mail: cb26@abnt.org.br
Comitês ISO/TC relacionados:
Categoria: O - membro observador | P - membro participante
- Membro - P: ISO/TC 76, ISO/TC 84, ISO/TC 121, ISO/TC 150, ISO/TC 157, ISO/TC 194, ISO/TC 198
- Membro - O: ISO/TC 106, ISO/TC 168, ISO/TC 170, ISO/TC 173
- XML in Clinical Research and Healthcare Industries (XML Coverpages) - Excelente página com MUITOS links para outras sites de órgãos de normatização e comitês, inclusive os do alto desta página, com informações sobre tendências e atualidades na utilização e desenvolvimento de normas baseadas em uma codificação utilizando a sintaxe XML.
- Clinical LOINC tutorial, Stan Huff, March 21, 2005
- Utilization of LOINC in the Partners Clinical Data Repository, Christine Raine & Elizabeth King, September 26, 2005
- Use of Intelligent Mapper with CPT to Optimize Radiology Mapping (AMIA presentation), Dan Vreeman & Clem McDonald, October, 2005
- LOINC & RELMA Tutorial #1, Clem McDonald, Aukland & Brisbane, November 2005
- LOINC & RELMA Tutorial #2, Clem McDonald, Aukland & Brisbane, November 2005
- Laboratory LOINC tutorial, Clem McDonald & Jim Case, December 5, 2005
- MeSH - Medical Subject Headings - Files Available to Download
- Documentação em CORBA Med
- Documentação CORBA MTM
1) Entrada de um Descritor MeSH em XML
<?xml version="1.0"?>
<!DOCTYPE DescriptorRecordSet SYSTEM "desc2006.dtd">
<DescriptorRecordSet>
<DescriptorRecord DescriptorClass = "1">
<DescriptorUI>D000001</DescriptorUI>
<DescriptorName>
<String>Calcimycin</String>
</DescriptorName>
<DateCreated>
<Year>1974</Year>
<Month>11</Month>
<Day>19</Day>
</DateCreated>
<DateRevised>
<Year>2003</Year>
<Month>07</Month>
<Day>30</Day>
</DateRevised>
<DateEstablished>
<Year>1984</Year>
<Month>01</Month>
<Day>01</Day>
</DateEstablished>
<ActiveMeSHYearList>
<Year>2004</Year>
<Year>2005</Year>
<Year>2006</Year>
</ActiveMeSHYearList>
<AllowableQualifiersList>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000008</QualifierUI>
<QualifierName>
<String>administration &
dosage</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>AD</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000009</QualifierUI>
<QualifierName>
<String>adverse effects</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>AE</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000031</QualifierUI>
<QualifierName>
<String>analogs & derivatives</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>AA</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000032</QualifierUI>
<QualifierName>
<String>analysis</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>AN</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000037</QualifierUI>
<QualifierName>
<String>antagonists &
inhibitors</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>AI</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000096</QualifierUI>
<QualifierName>
<String>biosynthesis</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>BI</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000097</QualifierUI>
<QualifierName>
<String>blood</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>BL</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000134</QualifierUI>
<QualifierName>
<String>cerebrospinal fluid</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>CF</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000138</QualifierUI>
<QualifierName>
<String>chemical synthesis</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>CS</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000145</QualifierUI>
<QualifierName>
<String>classification</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>CL</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000176</QualifierUI>
<QualifierName>
<String>diagnostic use</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>DU</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000191</QualifierUI>
<QualifierName>
<String>economics</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>EC</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000266</QualifierUI>
<QualifierName>
<String>history</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>HI</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000276</QualifierUI>
<QualifierName>
<String>immunology</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>IM</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000302</QualifierUI>
<QualifierName>
<String>isolation & purification</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>IP</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000378</QualifierUI>
<QualifierName>
<String>metabolism</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>ME</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000493</QualifierUI>
<QualifierName>
<String>pharmacokinetics</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>PK</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000494</QualifierUI>
<QualifierName>
<String>pharmacology</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>PD</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000506</QualifierUI>
<QualifierName>
<String>poisoning</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>PO</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000528</QualifierUI>
<QualifierName>
<String>radiation effects</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>RE</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000592</QualifierUI>
<QualifierName>
<String>standards</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>ST</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000600</QualifierUI>
<QualifierName>
<String>supply & distribution</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>SD</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000627</QualifierUI>
<QualifierName>
<String>therapeutic use</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>TU</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000633</QualifierUI>
<QualifierName>
<String>toxicity</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>TO</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000652</QualifierUI>
<QualifierName>
<String>urine</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>UR</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000737</QualifierUI>
<QualifierName>
<String>chemistry</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>CH</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000744</QualifierUI>
<QualifierName>
<String>contraindications</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>CT</Abbreviation>
</AllowableQualifier>
<AllowableQualifier>
<QualifierReferredTo>
<QualifierUI>Q000819</QualifierUI>
<QualifierName>
<String>agonists</String>
</QualifierName>
</QualifierReferredTo>
<Abbreviation>AG</Abbreviation>
</AllowableQualifier>
</AllowableQualifiersList>
<HistoryNote>91(75); was A 23187 1975-90 (see under ANTIBIOTICS
1975-83)
</HistoryNote>
<OnlineNote>use CALCIMYCIN to search A 23187 1975-90
</OnlineNote>
<PublicMeSHNote>91; was A 23187 1975-90 (see under ANTIBIOTICS
1975-83)
</PublicMeSHNote>
<PreviousIndexingList>
<PreviousIndexing>Antibiotics (1973-1974)</PreviousIndexing>
<PreviousIndexing>Carboxylic Acids (1973-1974)</PreviousIndexing>
</PreviousIndexingList>
<TreeNumberList>
<TreeNumber>D03.438.221.173</TreeNumber>
</TreeNumberList>
<RecordOriginatorsList>
<RecordOriginator>NLM</RecordOriginator>
<RecordMaintainer>SYSTEM</RecordMaintainer>
<RecordAuthorizer>NLM</RecordAuthorizer>
</RecordOriginatorsList>
<ConceptList>
<Concept PreferredConceptYN="Y">
<ConceptUI>M0000001</ConceptUI>
<ConceptName>
<String>Calcimycin</String>
</ConceptName>
<ConceptUMLSUI>C0000699</ConceptUMLSUI>
<CASN1Name>4-Benzoxazolecarboxylic acid,
5-(methylamino)-2-
((3,9,11-trimethyl-8-(1-methyl-2-oxo-2-
(1H-pyrrol-2-yl)ethyl)-1,7-dioxaspiro(5.5)undec-2-yl)methyl)-,
(6S-(6alpha(2S*,3S*),8beta(R*),9beta,11alpha))-</CASN1Name>
<RegistryNumber>52665-69-7</RegistryNumber>
<ScopeNote>An ionophorous, polyether antibiotic
from Streptomyces chartreusensis. It binds and transports cations across
membranes and uncouples oxidative phosphorylation while inhibiting ATPase
of rat liver mitochondria. The substance is used mostly as a biochemical
tool to study the role of divalent cations in various biological systems.
</ScopeNote>
<SemanticTypeList>
<SemanticType>
<SemanticTypeUI>T109</SemanticTypeUI>
<SemanticTypeName>Organic Chemical</SemanticTypeName>
</SemanticType>
<SemanticType>
<SemanticTypeUI>T195</SemanticTypeUI>
<SemanticTypeName>Antibiotic</SemanticTypeName>
</SemanticType>
</SemanticTypeList>
<PharmacologicalActionList>
<PharmacologicalAction>
<DescriptorReferredTo>
<DescriptorUI>D000900</DescriptorUI>
<DescriptorName>
<String>Anti-Bacterial
Agents</String>
</DescriptorName>
</DescriptorReferredTo>
</PharmacologicalAction>
<PharmacologicalAction>
<DescriptorReferredTo>
<DescriptorUI>D007476</DescriptorUI>
<DescriptorName>
<String>Ionophores</String>
</DescriptorName>
</DescriptorReferredTo>
</PharmacologicalAction>
</PharmacologicalActionList>
<ConceptRelationList>
<ConceptRelation RelationName="NRW">
<Concept1UI>M0000001</Concept1UI>
<Concept2UI>M0353609</Concept2UI>
</ConceptRelation>
</ConceptRelationList>
<TermList>
<Term ConceptPreferredTermYN="Y"
IsPermutedTermYN="N" LexicalTag="NON" PrintFlagYN="Y"
RecordPreferredTermYN="Y">
<TermUI>T000002</TermUI>
<String>Calcimycin</String>
<DateCreated>
<Year>1999</Year>
<Month>01</Month>
<Day>01</Day>
</DateCreated>
<ThesaurusIDlist>
<ThesaurusID>NLM (1975)</ThesaurusID>
</ThesaurusIDlist>
</Term>
</TermList>
</Concept>
<Concept PreferredConceptYN="N">
<ConceptUI>M0353609</ConceptUI>
<ConceptName>
<String>A-23187</String>
</ConceptName>
<ConceptUMLSUI>C0878412</ConceptUMLSUI>
<RegistryNumber>0</RegistryNumber>
<SemanticTypeList>
<SemanticType>
<SemanticTypeUI>T109</SemanticTypeUI>
<SemanticTypeName>Organic Chemical</SemanticTypeName>
</SemanticType>
<SemanticType>
<SemanticTypeUI>T195</SemanticTypeUI>
<SemanticTypeName>Antibiotic</SemanticTypeName>
</SemanticType>
</SemanticTypeList>
<ConceptRelationList>
<ConceptRelation RelationName="NRW">
<Concept1UI>M0000001</Concept1UI>
<Concept2UI>M0353609</Concept2UI>
</ConceptRelation>
</ConceptRelationList>
<TermList>
<Term ConceptPreferredTermYN="Y"
IsPermutedTermYN="N" LexicalTag="LAB" PrintFlagYN="N"
RecordPreferredTermYN="N">
<TermUI>T000001</TermUI>
<String>A-23187</String>
<DateCreated>
<Year>1990</Year>
<Month>03</Month>
<Day>08</Day>
</DateCreated>
<SortVersion>A A 23187</SortVersion>
<ThesaurusIDlist>
<ThesaurusID>NLM (1991)</ThesaurusID>
</ThesaurusIDlist>
</Term>
<Term IsPermutedTermYN="Y"
LexicalTag="LAB">
<TermUI>T000001</TermUI>
<String>A 23187</String>
</Term>
<Term ConceptPreferredTermYN="N"
IsPermutedTermYN="N" LexicalTag="NON" PrintFlagYN="N"
RecordPreferredTermYN="N">
<TermUI>T000003</TermUI>
<String>Antibiotic A23187</String>
<DateCreated>
<Year>1990</Year>
<Month>03</Month>
<Day>08</Day>
</DateCreated>
<ThesaurusIDlist>
<ThesaurusID>NLM (1991)</ThesaurusID>
</ThesaurusIDlist>
</Term>
<Term IsPermutedTermYN="Y"
LexicalTag="NON">
<TermUI>T000003</TermUI>
<String>A23187, Antibiotic</String>
</Term>
<Term ConceptPreferredTermYN="N"
IsPermutedTermYN="N" LexicalTag="LAB" PrintFlagYN="N"
RecordPreferredTermYN="N">
<TermUI>T000004</TermUI>
<String>A23187</String>
<DateCreated>
<Year>1974</Year>
<Month>11</Month>
<Day>11</Day>
</DateCreated>
<ThesaurusIDlist>
<ThesaurusID>UNK (19XX)</ThesaurusID>
</ThesaurusIDlist>
</Term>
</TermList>
</Concept>
</ConceptList>
</DescriptorRecord>
</DescriptorRecordSet>
Uma mensagem de Registro de Paciente completa em HL7 (Exemplo de Kenneth S. Rubin)
MSH|^~\&|REGADT|MCM|IFENG||199112311501||ADT^A04|000001|P|2.4|||
EVN|A04|199901101500|199901101400|01||199901101410
PID|||191919^^^GENHOS^MR~371-66-9256^^^USSSA^SS|253763|MASSIE^JAMES^A||
19560129|M|||171 ZOBERLEIN^^ISHPEMING^MI^49849^""^||(900)485-5344|(900)485-5344||S|C|
10199925^^^GENHOS^AN|371-66-9256||
NK1|1|MASSIE^ELLEN|SPOUSE|171 ZOBERLEIN^^ISHPEMING^MI^49849^""^|
(900)485-5344|(900)545-1234~(900)545-1200|
EC1^FIRST EMERGENCY CONTACT
NK1|2|MASSIE^MARYLOU|MOTHER|300 ZOBERLEIN^^ISHPEMING^MI^49849^""^|(900)485-5344|(900)545-1234~(900)545-1200|
EC2^SECOND EMERGENCY CONTACT
PV1||O|O/R||||0148^ADDISON,JAMES|0148^ADDISON,JAMES|0148^ADDISON,JAMES|AMB||
|||||0148^ADDISON,JAMES|S|1400|A|||||||||||||||||||GENHOS|||||199501101410|
PV2||||||||199901101400||||||||||||||||||||||||||199901101400
OBX||ST|1010.1^BODY WEIGHT||62|kg|||||F
OBX||ST|1010.1^HEIGHT||190|cm|||||F
DG1|1|19||BIOPSY||00|
GT1|1||MASSIE^JAMES^""^""^""^""^||171 ZOBERLEIN^^ISHPEMING^MI^49849^""^|
(900)485-5344|(900)485-5344
||||SE^SELF|371-66-925||||MOOSES AUTO CLINIC|171 ZOBERLEIN^^ISHPEMING^MI^49849^""|
(900)485-5344|
IN1|0|0|BC1|BLUE CROSS|171 ZOBERLEIN^^ISHPEMING^M149849^""^||
(900)485-5344|90||||||50 OK|
Abaixo repetimos a mesma mensagem, agora comentada:
Cabeçalho e descritor de evento
MSH|^~\&|REGADT|MCM|IFENG||199112311501||ADT^A04|000001|P|2.4|||<cr>
EVN|A04|199901101500|199901101400|01||199901101410<cr>
Identificador do Paciente
PID|||191919^^^GENHOS^MR~371-66-9256^^^USSSA^SS|253763|MASSIE^JAMES^A||19560129|
M|||171 ZOBERLEIN^^ISHPEMING^MI^49849^""^||(900)485-5344|(900)485-5344||S|C|
10199925^^^GENHOS^AN|371-66-9256||<cr>
Dados de Parentes Próximos
NK1|1|MASSIE^ELLEN|SPOUSE|171 ZOBERLEIN^^ISHPEMING^MI^49849^""^|(900)485-5344
|(900)545-1234~(900)545-1200|EC1^FIRST EMERGENCY CONTACT<cr>
NK1|2|MASSIE^MARYLOU|MOTHER|300 ZOBERLEIN^^ISHPEMING^MI^49849^""^|(900)485-5344
|(900)545-1234~(900)545-1200|EC2^SECOND EMERGENCY CONTACT<cr>
Informação sobre a Consulta
PV1||O|O/R||||0148^ADDISON,JAMES|0148^ADDISON,JAMES|0148^ADDISON,JAMES|AMB|
||||||0148^ADDISON,JAMES|S|1400|A|||||||||||||||||||GENHOS|||||199501101410|<cr>
PV2||||||||199901101400||||||||||||||||||||||||||199901101400<cr>
Dados biométricos
OBX||ST|1010.1^BODY WEIGHT||62|kg|||||F<cr>
OBX||ST|1010.1^HEIGHT||190|cm|||||F<cr>
Diagnostico
DG1|1|19||BIOPSY||00|<cr>
Plano de Saúde
GT1|1||MASSIE^JAMES^""^""^""^""^||171 ZOBERLEIN^^ISHPEMING^MI^49849^""^|(900)485-5344|(900)485-5344||||SE^SELF|
371-66-925||||MOOSES AUTO CLINIC|171 ZOBERLEIN^^ISHPEMING^MI^49849^""|(900)485-5344|<cr>
IN1|0|0|BC1|BLUE CROSS|171 ZOBERLEIN^^ISHPEMING^M149849^""^||
(900)485-5344|90||||||50 OK|<cr>



